Clean Room Injection Molding Service
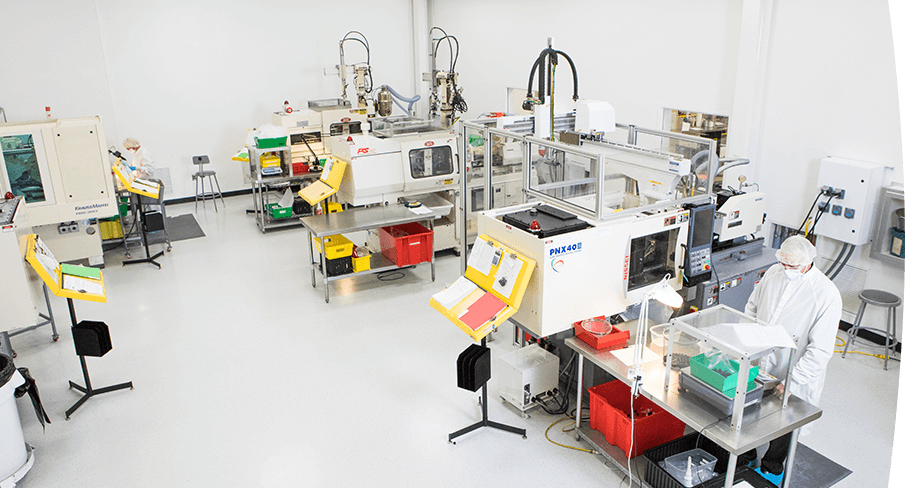
The company strictly implements the ISO9001 quality system, equipped with advanced clean room injection molding machines and professional processors. These machines have a special processing system to ensure dust-free quality and high performance standards in a constant temperature and humidity environment. Holly also has a professional team that can shape, assemble and package your products in our clean room environment. This not only ensures that your part stays clean, but also frees up time to focus on marketing and sales. The clean room injection workshop is mainly used in medical, optical, electronic and other industries. Holly is your value-added clean room injection molding partner.
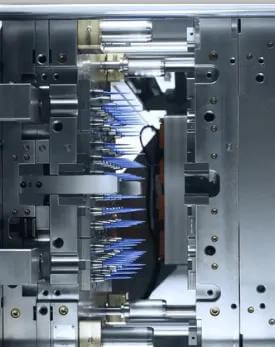
Tip Mold

Medical Tube Mold

Medical Graduate Mold

Cap Mold
Clean Room Injection Molding Parts
Holly clean room has advanced mold manufacturing equipment, such as high-speed CNC, precision wire cutting, precision EDM, etc., which are strict guarantees of molding accuracy. We have the ability to provide tailor-made products, including medical accessories, bottle caps and medical accessories.

Tips for laboratory
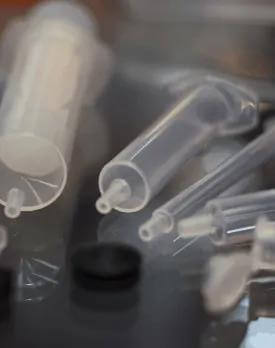
Medical Syringe Shell
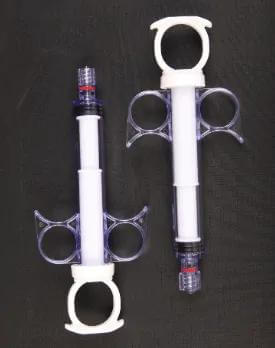
Syringe Medical

Medical Connector
China Clean Room Injection Molding Manufacturer FAQ


Clean Room Injection Molding – Ultimate FAQ Guide
Clean room injection molding is widely used in many industries such as optics, electronics, precision manufacturing, medicine, and health. According to the precision requirements of the industry and production process, the clean room of injection molding changes greatly, and the purification level needs to be analyzed case by case.
The company cooperates with many scientific research and medical institutions, has the rich practical experience, and can produce products that meet the standards. As a professional clean room injection molding manufacturer, we have more than 20 years of production experience. Holly serves you wholeheartedly.
You can inquire about any questions you want to know about clean room injection molding. like:
- What is clean room injection molding?
- What are the requirements for clean room injection molding?
- What is Holly’s clean room injection molding standard?
For further communication, please feel free to contact the Holly team. Here is a list of frequently asked questions about clean room injection molding.
1. What products are the clean room injection molding processes usually used for?
Typical applications of clean room injection molding processes in the industry are as follows:
- Electronic product manufacturing.
- Medicine packaging.
- Manufacturing of medical plastic products.
- Automobile manufacturing industry.
2. What is the composition of the clean room injection molding room?
A clean room (area) is the working environment required for the production and inspection of medical devices. During production, functional rooms should be included corresponding to the production process, such as injection rooms, drying rooms, etc. It should include functional rooms related to testing, such as positive control room, sterile room, and microbial limit room; Production auxiliary function room, such as laundry room, sanitary warehouse, etc. Various functions are connected with the buffer transition channel.
3. What are the main factors that affect the cleanliness of the clean room (area)?
- The characteristics of medical devices.
- Medical device production technology.
- Personnel, facilities, sanitary requirements, and utensils.
- Atmospheric Environment.
4. How to clean, disinfect and disinfect the clean room (area)?
Commonly used surface disinfection and sterilization methods include ultraviolet lamp irradiation, ozone contact, gas fumigation such as peroxyhexanoic acid, alkylene oxide, and disinfectant spraying.
Disinfection and sterilization is the main method to get rid of microbial contamination, but to ensure the thoroughness of disinfection and sterilization, manufacturers must formulate disinfection and sterilization procedures and regularly verify their effectiveness.
5. What are the common defects in the production of medical syringes when using a clean room?
When using a clean room, the common defects in the production of medical syringes are as follows:
Defects such as shrinkage, white spots, wave marks, and flashing appear on the surface of the needle cover of medical syringes.
6. What are the characteristics of a clean room in the electronics manufacturing industry?
The characteristics of clean rooms in the electronics manufacturing industry are as follows:
1) Clean rooms in the electronics manufacturing industry have extremely strict requirements for static electricity, especially humidity. Under normal circumstances, the temperature of the electronics workshop should be controlled at around 22, and the relative humidity should be controlled between 50-60% (special clean workshops have relevant regulations on temperature and humidity). Because an excessively dry workshop is prone to static electricity, which leads to damage to the CMOS integration.
2) The noise level (empty state) of the class 10000 clean room of the electronics manufacturing plant: no more than 65db (a).
3) The filling rate of the vertical flow clean room of the clean room of the electronics manufacturing plant should not be less than 60%, and the horizontal one-way flow clean room should not be less than 40%, otherwise, it will be a partial one-way flow.
4) The static pressure difference between the clean room and the outdoor. It should not be less than 10Pa, and the static pressure difference between clean and non-clean areas with different air cleanliness should not be less than 5Pa.
7. What are the basic characteristics of a biopharmaceutical clean room?
The basic characteristics of the biopharmaceutical clean room injection molding room are as follows:
- The decontamination room of biopharmaceuticals must take dust particles. Microorganisms are used as environmental control objects.
- There are four levels of store cleaning: 100 or 10000, local 100, 1000, 10000, and 30000.
- Clean room temperature: no special requirements, at 18 ~ 26 degrees, relative humidity controlled at 45% ~ 65%.
- Pollution Control in the clean workshop of biopharmaceuticals: pollution source control, decentralized process control, and cross-contamination control.
- The key technology of the clean room in the biopharmaceutical industry is mainly to control dust and microorganisms as pollutants. Microbes are the most important part of the clean room environment control.

8. What are the requirements for a clean room workshop for pharmaceutical packaging?
The requirements for the clean room workshop of pharmaceutical packaging are as follows:
- Provide the level of air purification required for production, and regularly detect and record the number of air dust particles and living microorganisms in the purification project of the packaging workshop. There should be no static pressure difference between the levels of packaging workshops.
- The temperature and relative humidity of the purification project of the packaging workshop should be compatible with the requirements of the production process.
- The production areas of penicillin, hypersensitivity drugs, and antitumor drugs should be equipped with independent air conditioning systems, and the exhaust gas should be purified.
- For the dust room, an effective dust-catching device should be installed to prevent cross-contamination of dust.
- For auxiliary production rooms such as storage, ventilation facilities, temperature and humidity, they should meet the requirements of pharmaceutical production and packaging.
9. What is clean room injection molding?
Clean room molding is the creation of plastic parts in a special room to reduce the risk of dust or other particle contamination. Clean room molding is widely used in medical, pharmaceutical, aerospace, military, biotechnology and other industries.
There are 9 “levels” of clean rooms, level 1 is the most sterile, and level 9 is the least sterile. In a clean room, most plastic injection molding is carried out in a class 7 or 8 clean room. This is a standard required by any portable device.

10. What are the requirements for clean room injection molding?
Positive airflow: In order to ensure the purity of the air and maintain a strict environment and particle count, the level 7 and 8 injection molding clean rooms use positive airflow to ensure specific particulate matter.
Wear protective clothing: Clean room injection molding is a restricted area. Authorized personnel must be allowed to enter the clean room and must cover their entire body, including shoe covers, a full set of protective clothing and hair covers. All clothing must be replaced immediately after being contaminated.
Motor: In order to avoid more particles in the air, in a clean room injection molding environment, a motor replaces a hydraulic press.
Packaging requirements: Some corrugated packaging will produce extra particulate matter, so it is not allowed to be used in a clean room.
Coated cardboard or plastic packaging is the most common. The Chemmar clean room has been used in a wide range of custom molding applications for more than 20 years, and it strictly meets the requirements of the industry’s ISO 14644 requirements. It is especially suitable for medical components that require compliance with ISO 13485 standards. Through engineering stages, such as product design, project management, mold construction, material selection and product testing, it provides a competitive advantage for clean room molded plastics.

11. What is Holly’s clean room injection molding standard?
Clean room injection molding standards are as follows:
- Scientific molding method
- Tight tolerances
- Flame retardant
- Antifouling
- anti-static
- Anti-skid
- Antibacterial
- Antioxidants
- Compressed air filtration system
- Vacuum bag sealing machine
12. When a customer requests injection molding in a clean room, what service does Holly provide?
- Product design
- Design review (DFM/mold flow analysis, tolerance analysis, feasibility study)
- 3D printing or prototype mold
- Mould.
- Injection
- Mass production
- Tool maintenance.
- Technical support and service.

13. What must the clean room injection molding workers strictly pay attention to?
One point that injection molders must strictly pay attention to is:
- Quality control system.
- Process documentation.
- Working environment
- Risk management.
- Operation training
14. What kind of medical products need to be injection molded in a medical clean room?
The medical parts that need to be injection molded in a medical clean room are as follows:
- Medical equipment housing
- Surgical instruments
- Implant
- Emergency room products
- Fluid delivery device
- Fluid delivery container
- Heart products
- Send blood room
- Optical grade lens
- Dental products
- Medical equipment housing
- Surgical instruments
- Urgently plantable
- Fluid delivery device
- Fluid delivery container
- Heart products

15. What plastic materials are commonly used in medical clean room injection molding?
Plastic materials commonly used in injection molding in medical cleanrooms are as follows:
- PC / ABS
- Abdominal muscles
- Acetal
- HDPE
- LDPE nylon stockings
- TPE
16. What to consider for a clean room?
- Quality control systems, such as HEPA filters and hoods, monitor, manage. Remove particles and dust, otherwise, it may cause the required number of contaminated particles to fall outside the specifications of the ISO 7 and ISO 8 clean room standards.
- Process documentation, physically tracking the process life cycle, die drawing cycle, and equipment/product testing, so that the controlled conditions can be verified at any time in the case of repeated cycles, routine inspections, customer requirements, or program deviations.
- Work environment control to detect the purity of air quality and regular, comprehensive equipment inspections to discover and deal with contaminants generated during production, such as sprayed parts, peeled or shaved bottom edges, and microscopic plastic parts particles. In addition, if machine maintenance is not done well, pollutants may be introduced. For example, ingredients with too much oil can be particularly harmful.
- During maintenance, if you do not do well, you can introduce contacts. For example, parts that are overly lubricated may be particularly prone to fall off.
- Carry out risk management by implementing specific analysis modules such as Fault Tree Analysis (FTA), Failure Mode and Impact Analysis (FEMA), Hazard Analysis and Critical Control Points (HACCP). Understanding and evaluating microbiological risks helps injection molding plants determine acceptable limits and develop consistent pollution control assessments during the production process.
- The traceability requirements for implantable medical products, non-implantable medical devices, life-sustaining devices used outside medical facilities, and all other complex applications regulated by FDA that intend to stay in the human body for more than one year, these applications The failures of this group can also have serious and adverse health consequences.
- The machine operator must fully follow the procedures in the clean room, because corner cutting is unacceptable. In addition, they must strictly observe the wearing of protective equipment such as gloves, gowns, masks and boots to protect the clean room environment and injection molded parts from contamination.
17. What is the classification of clean rooms?
In addition to ISO and CFR standards, clean room injection molding environments are also classified, usually classified as ISO 7 or ISO 8. The former refers to the clean room containing 10,000 pollution particles per cubic foot, and the latter refers to the clean room containing 100,000 pollution particles per cubic foot. The type of clean room used for production is determined by the specific product and/or customer requirements.

18. How to maintain clean room standards?
Complex medical applications need to protect equipment and equipment from dust, chemical vapors, aerosol particles, airborne microorganisms, and other pollutants, which can compromise product quality, integrity, and safety.
Injection molders responsible for providing services to medical manufacturers are obliged to comply with strict agreements. In addition to operating in a clean room that complies with ISO 13485:2016 certification and 21 CFR 820 (the current quality standard). MedAccred certification ensures that medical OEMs meet unparalleled benchmarks in terms of production and quality standards.
19. Do you want to ensure the smooth serial production of high-end injection molded parts under clean production conditions?
If you need clean room injection molding services, Holly provides corresponding clean room solutions: Only first-class: everything from stand-alone machines to fully automated turnkey systems comes from expert sources. Used directly in your clean room, docked with the clean area, or as a separate clean room unit. Holly’s advanced technology and in-depth expertise work hand in hand. This is a kind of dust-free workshop production, nothing is satisfactory.
20. Why choose Holly as a clean room injection molding supplier?
1) Standards and guidelines
Protect injection molded parts from airborne pollutants such as dust particles, bacteria, or viruses? To this end, it is necessary to limit the negative effects produced during the production process and prevent them from contacting the product.
A high-quality ventilation system can achieve this in practice. Take air conditioning as an example. The core rule that defines a clean room is the EN ISO 14644 standard. This can be supplemented by further EN ISO, VDI, and VDA standards, GMP (Good Manufacturing Practice) specifications, EU and US FDA (Federal Drug Administration) directives, and personal and corporate standards.
2) Qualified and verified
In order to meet the requirements of ISO 13485 and GMP, we provide comprehensive qualification documents for all talents. It includes standard machine configuration, operation manual, function description, maintenance cycle, cleaning instructions, spare parts list, establishment plan, and circuit diagram. If necessary, the inspection log can be used as proof of machine performance. In order to maintain the repeatability of the injection molding machine, you can sign a service contract with us to ensure regular re-verification.
3) Important: cleaning operation
To maintain a clean production environment, clean rooms and all production facilities must be cleaned and disinfected regularly. The injection molding technology we provide is easy to clean, which improves operating efficiency accordingly. In addition, the specific hygiene measures of the staff are also decisive. Special work clothes and work instructions (cleaning procedures before and after work in the clean room) are required, without exception.























